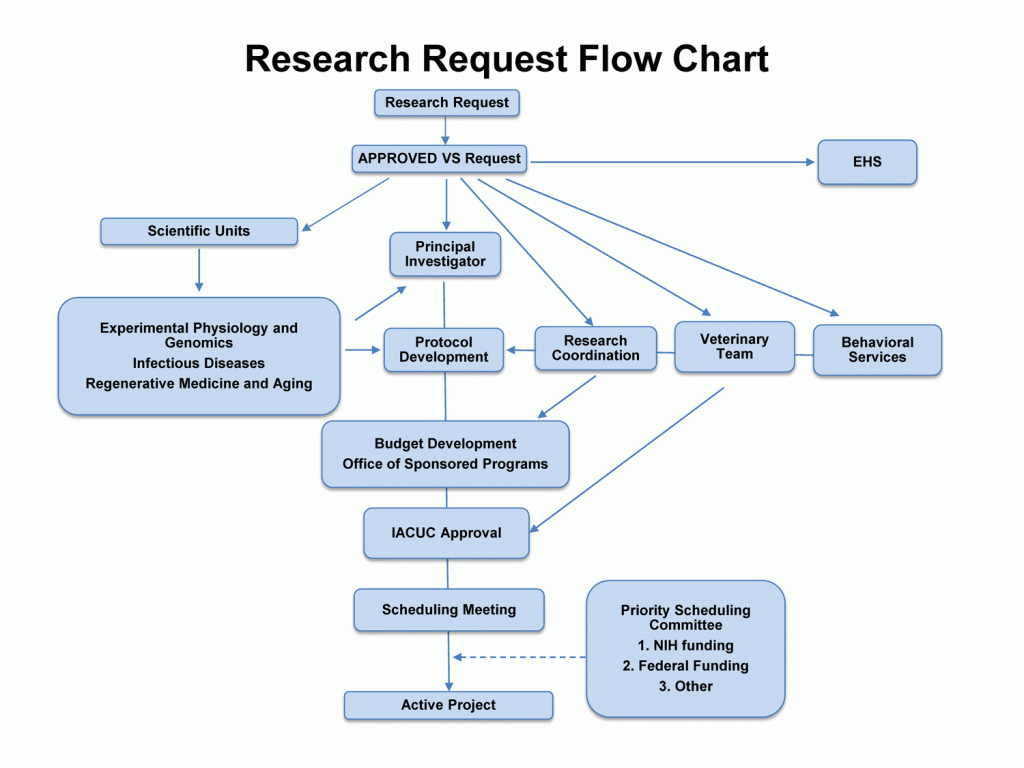
Please see to outline below regarding the research resources request process.
Research Resources Flow Chart Legend
-
Research Request
Requests to use SNPRC resources are made via the SNPRC website. Upon submission, the request is automatically e-mailed to Research Coordination staff. A tracking number is assigned (VS #) and the requestor is e-mailed an acknowledgement of the request along with the assigned VS #; the email is generally sent within 48 hours of receipt of the Research Request.
-
Approved VS Request
Each request is reviewed by a species-specific committee to determine the availability of resources required to further consider the request. Recommendation of approval for simple, straightforward projects is usually obtained in one business day. More complex projects that have a greater impact on available resources can be deferred for discussion at the next monthly species committee meeting. Questions from committee members are forwarded to the requestor as they arise. The recommendation of the species advisory committee is forwarded to the Research Advisory Committee and the Director for final approval.
-
Environment, Health, and Safety (EHS)
The Director of EHS is notified if an approved request will use bio-hazardous materials.
-
Principal Investigator (PI)
The requestor (PI) is notified when the request is initially recommended for approval and may receive a rough “ballpark” estimate of costs for the project, if costs were requested at the time of the research request.
-
Scientific Units (SU)
The Leaders of one of the SNPRC Scientific Units are notified in order to provide the expertise needed to work with the Principal Investigator. The SNPRC provide expertise in the following areas: Infectious Diseases; Experimental Physiology and Genomics; and Regenerative Medicine and Aging. The Unit Leaders may refer the request to another Core Scientist within the SNPRC.
-
Research Coordinator
A Research Coordinator is assigned to a VS Request early on, especially when the PI requests information on costs. The Research Coordinator assists with generating procedure costs and budgets, develops study time lines, and communicates with the PI and with the veterinary staff of Veterinary Resources and Research Support with regard to study activity.
-
Veterinary Team
A Veterinary Team consists of veterinarians, pathologists, supervisors and technical staff. Each project is conducted by a single assigned team to ensure consistency and continuity.
-
Behavioral Services
Behavioral Services reviews research requests to determine if behavioral input is needed. Behavioral Services may recommend changes to study design elements such as housing, training, and enrichment.
-
Protocol Development
There are times when the scope of the protocol will depend on the cost of performing the work. Along with the Veterinary Team, PI and SU Leaders, Research Coordinators are intimately involved with the developing study outlines, budgeting, initiating contracts, and generating time lines for projects.
-
Budget Development and Office of Sponsored Programs (OSP)
If the scope of the project needs to be adjusted (e.g., by increasing or decreasing the number of animals or procedures), the Research Coordinator can work with the PI to design an affordable study. The Research Coordinator may contact other staff in Veterinary Resources and Research Support for input on the budget (e.g., to determine if work after-hours, specialized procedures or other issues need to be considered). Once the PI approves the final version, the Research Coordinator sends the budget to Office of Sponsored Programs for inclusion in the contract or proposal. Upon receipt of the final signed contract or award notice, the Research Coordination Staff will note that the project or purchase is “funded” in the project documentation.
-
IACUC Approval
The PI works with the assigned veterinarian to finalize the IACUC application. When the protocol is approved by the IACUC Committee, it advances in the queue of projects. There are two major milestones that must be met before a project can be initiated. The IACUC must approve the study protocol and the project must be funded.
-
Scheduling Meeting
Meetings with all veterinarians, Assistant Director of Research Resources, and the Manager of Research Coordination take place biweekly. All projects that are ready to be scheduled are reviewed for completeness and in order to resolve any remaining issues.
-
Priority Scheduling Committee
The Priority Scheduling Committee is chaired by the Assistant Director of Research Resources. The other members of the Committee are the Associate Director for Research, the Associate Director for Veterinary Resources and Research Support, one senior staff member who has no active animal protocols, and one non-voting scientist with animal study experience. The Committee convenes as necessary to determine which projects in the queue take priority for scheduling procedures. Source of funding and amount of time in the queue are examples of factors taken into consideration by the Committee. First priority is given to NIH supported projects.
-
Active Project
Once all of the necessary milestones are met, a project or purchase becomes active and the daily procedures and activities are managed by the veterinary staff of Veterinary Resources and Research Support. A Research Coordinator remains assigned to the project for the purpose of scheduling, scope and budget revision, procedure monitoring and documentation. The Research Coordinator also assists the Veterinary Team with communication and in-life study meetings. In addition, all active projects are discussed at a biweekly meeting between key staff of the Primate Center (veterinarians, pathologists, staff scientists, supervisors, research coordinators, training coordinator, animal resources manager, and the finance group). Costs of each active project also are monitored by Research Coordination.